Determining the molecular mechanism underpinning vascular inflammation in resistance to radiotherapy
Primary supervisor: Kairbaan Hodivala-Dilke, Queen Mary University of London
Secondary supervisor: Rita Pedrosa, Queen Mary University of London
Tertiary supervisor: Erik Sahai, The Francis Crick institute
Project
This exciting PhD project will use state-of-the-art technologies and models to explore pollutant microparticles and tobacco induced vascular inflammatory changes in lung cancer and determine the mechanisms by which these affect radiotherapy resistance. The project aligns with ongoing studies in the theme of Overcoming Radioresistance: Novel combination strategies.
Lung cancer: Lung cancer is the most common cancer worldwide, and accounts for over 12% of all diagnosed cancers. Currently, lung cancer is the leading reason for cancer-related deaths.
Cancer Inflammation includes endothelial cells: Long-term exposure to pollutant microparticles and tobacco smoking, is positively associated with inflammation which can predispose to lung cancer, and with poorer therapeutic responses [1,2]. Recent advances have shown that endothelial cells, that line blood vessels, also play a part in inflammatory responses [3,4] , and our work has shown that genetic modulation of endothelial cells can affect radioresistance [5].
Radiotherapy: Radiotherapy is part of the standard-of-care treatment for human lung cancer, but can lead to cancer inflammation, and to radiotherapy resistance in around 50% of treated patients. The molecular regulation of inflammation by endothelial cells in radiotherapy resistance is poorly understood.
State-of-the-art model of lung cancer: Primary lung cancer in humans usually presents as a single cancer nodule with clear signs of inflammation and vascularisation. The proposed PhD project will be based on experiments using our newly developed unifocal cancer model that has close-to-human characteristics in inflammation and is suitable for stereotactic focal radiotherapy (Figure 1A).
- Preliminary Data: The PhD project is based on our observations that radiotherapy can:
Enhance the frequency of a specialized sub-population of vascular immune modulatory endothelial cells, called iMECs (iM-Cap), uniquely expressing PD-L1 and enriched in inflammatory signatures (NF-Kb, IFNg, etc..) in mouse lung (Figure 1B) (Data adapted from Nolan, et al, Nature, 2022) - Affect the growth of mouse lung tumours that correlates with survival benefits in mice with KP but not CMT tumours. These data provide a model of radioresistant lung cancer.
- Induce an increase in the vascular inflammatory maker, VCAM, in endothelial cells associated with CMT but not KP tumours, suggesting that vascular inflammation is associated with radioresistance.
- Alters levels of effector memory CD4+ cells. and increased monocytic populations in CMT but not KP tumours, pointing towards a more immunosuppressive environment in the CMT model relevant to radiotherapy resistance.
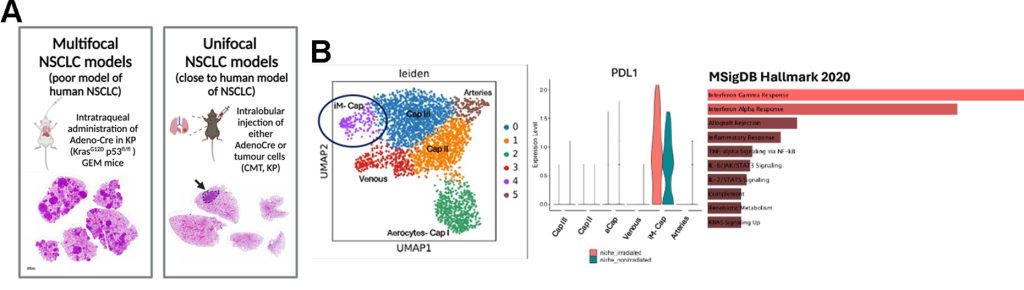
Figure 1. Preliminary data reveal vascular inflammatory features associated with radioresistance in lung cancer. (A) Development of unifocal, close to human mouse models of lung cancer. (B) scRNA sequencing analysis shows that vascular inflammatory iMEC (immunomodulatory microvascular endothelial cells) are enriched in lung after radiotherapy in mice. This iMEC population uniquely express PDL1 and present enriched inflammatory signatures compared with the other clusters of vascular endothelial cells in the mouse lung.
PhD Objectives
- Induce tobacco and pollution microparticle aggravated non-small cell lung cancer in our unifocal mouse models and treat with radiotherapy using small animal radiation research platform. Any changes in radiotherapy resistance will provide a model of pollutants or tobacco induced inflammation and associated radiotherapy resistance. Combination therapies including immunotherapy will also be tested alongside radiotherapy
- Using a combination of single cell transcriptomics, multiplex imaging and hi-plex flow cytometry we will define the molecular changes in the stromal and especially the endothelial compartment associated with vascular inflammation and radiotherapy resistance in the tumours produced in (1)
- Test for the presence of, and molecular profiles for iMECs in human lung cancer. This will define the human relevance of the PhD study
- Use murine and cell-based assays to functionally test the molecular targets identified in (2) and determine molecular mechanisms underpinning vascular inflammation in lung cancer radioresistance.
Candidate background
The successful candidate should have a good knowledge of lung cancer and the tumour microenvironment with an interest in understanding mechanisms of radioresistance
Potential Research Placements
- Erik Sahai, The Francis Crick Institute
- Alex Wang, Queen Mary University of London
References:
- Perdyan, A. & Jassem, J. Impact of Tobacco Smoking on Outcomes of Radiotherapy: A Narrative Review. Curr Oncol 29, 2284-2300 (2022). https://doi.org/10.3390/curroncol29040186
- Lagunas-Rangel, F. A., Liu, W. & Schioth, H. B. Can Exposure to Environmental Pollutants Be Associated with Less Effective Chemotherapy in Cancer Patients? Int J Environ Res Public Health 19 (2022). https://doi.org/10.3390/ijerph19042064
- Amersfoort, J., Eelen, G. & Carmeliet, P. Immunomodulation by endothelial cells – partnering up with the immune system? Nat Rev Immunol 22, 576-588 (2022). https://doi.org/10.1038/s41577-022-00694-4
- Taguchi, K. et al. Tumor Endothelial Cell-Mediated Antigen-Specific T-cell Suppression via the PD-1/PD-L1 Pathway. Molecular cancer research : MCR 18, 1427-1440 (2020). https://doi.org/10.1158/1541-7786.MCR-19-0897
- Tavora, B. et al. Endothelial-cell FAK targeting sensitizes tumours to DNA-damaging therapy. Nature 514, 112-116 (2014). https://doi.org/10.1038/nature13541
