Combination of anti-GD2 Molecular Radiotherapy and γδ-T cells for the Enhanced Treatment of Childhood Bone Cancers
Primary supervisor: Jane Sosabowski, Queen Mary University of London
Secondary supervisor: Jonathan Fisher, University College London
Project
There are around 160 new osteosarcoma (OS) and 90 new Ewing sarcoma (EWS) diagnoses each year in the UK – primarily affecting children/young adults aged 10-24 – 20% of which are already metastatic. Relapse occurs in 80% of patients with metastatic disease, typically in the lungs, conferring dismal prognosis despite highly toxic chemotherapy and life-altering surgery. For OS patients with relapsed and/or metastatic disease, the 4-month event-free survival is only 10%, and in EWS, <30% of patients with metastases survive for 5 years.
Both tumour types typically express high levels of the disialoganglioside antigen GD2, prompting the development of anti-GD2 immunotherapies against both diseases [1,2]. Unfortunately, immunotherapy alone is unlikely to deliver durable remissions, and combinations with other modalities are needed. This project seeks to explore the combination of targeted radiotherapy with cellular immunotherapy.
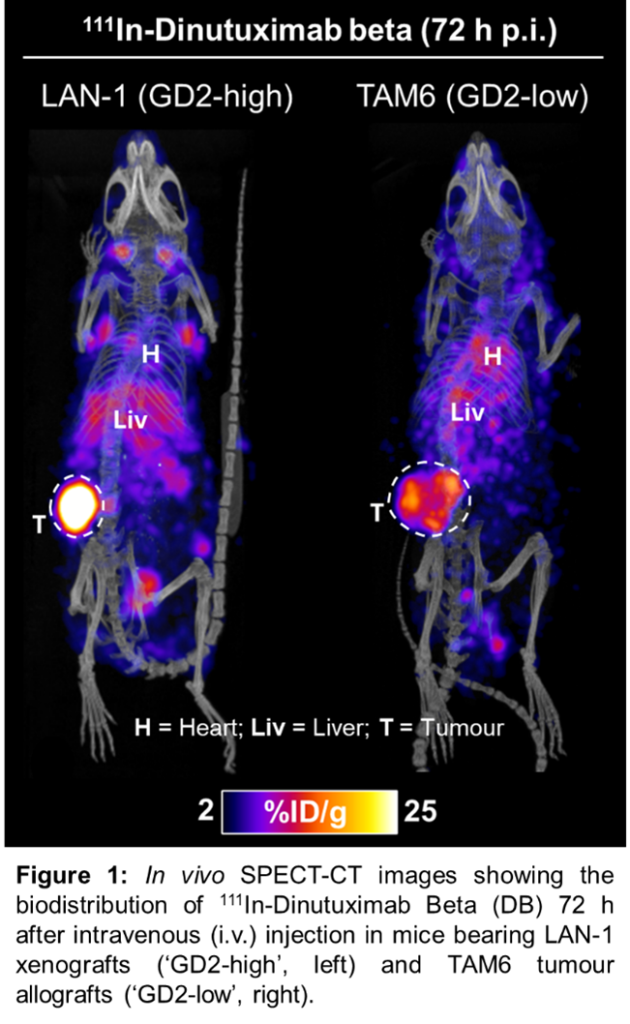
Molecular radiotherapy (MRT) – whereby cytotoxic radionuclides are delivered to tumours using targeting molecules – is an under-researched form of systemic radiation therapy currently attracting major pharma investment, and great interest in novel alpha-emitting radionuclides and combination therapies. We have developed and validated a radiotherapeutic agent, using an anti-GD2 antibody, Dinutuximab-beta (DB), allowing non-invasive in vivo imaging of GD2 tumour expression (Figure 1), and molecular radiotherapy – using various radionuclides. Therapeutic studies are currently ongoing.
There is emerging evidence that low-dose tumour irradiation (including MRT) in combination with cellular therapies (i.e. CAR T-cells) can have a synergistic effect resulting in improved tumour killing ability of the cellular therapy [3]. However, combination of MRT with conventional CAR-αβT cell therapy is extremely expensive, requiring individual patient CAR-T manufacture. Alternatively, gamma-delta (γδ) T cells are effector cells possessing potent innate anti-tumour cytotoxicity and improved safety profile in the allogeneic setting. γδT cells form part of the innate lymphoid stress surveillance system which responds to damaged, transformed or infected cells. Importantly, γδT cells have shown demonstrable activity against OS and EWS, which is enhanced in the context of anti-GD2 antibodies [1,2].
We hypothesise that exposure to MRT will sensitise bone tumours by upregulating stress markers known to be recognized by γδT cells – leading to improved cancer killing. We will investigate whether a) we can target EWS and OS with MRT, and b) whether low-dose MRT in combination with γδ T-cells can enhance EWS tumour killing. We will also investigate whether this combination could unlock the potential of radiotherapy to treat OS, which is typically highly resistant to radiotherapy when used in isolation.
We have access to a range of GD2 expressing EWS and OS models – including patient-derived cell lines – which we will use to evaluate the efficacy of our GD2-targeted radiotherapeutic: using 177Lu-/212Pb-DB for beta- and alpha-emitting MRT respectively. In vitro cell assays will be used to establish specific GD2-binding of 177Lu-/212Pb-DB in OS and EWS cells, as well as the radiobiological response to therapeutic doses (e.g. DNA damage, viability and survival). In vivo radionuclide imaging (Figure 1) of pre-clinical OS and EWS models will be performed to assess specific tumour binding.
Secondly, we will investigate the synergistic effect of ‘low-dose’ GD2-targeted radiotherapy, in combination with γδ T-cells, in in vitro models of EWS and OS: we will expose the tumour cells to varying doses of 177Lu-/212Pb-DB, washing and then adding γδT cells to find the optimal time point/dose/radionuclide for sensitisation. We hypothesise that synergising the direct effect of radiotherapy, sensitisation to innate γδT cell cytotoxicity and engagement of γδT cell antibody-dependent cytotoxicity will deliver superior tumour kill than either therapy alone. We will first trial this with EWS, before attempting the same with the typically radio-resistant OS. External beam radiotherapy will be used as a non-immunological comparison.
Candidate background
We are looking for candidates with a background in pharmacology/biology/(bio)chemistry and an interest in expanding their molecular biology and immunology knowledge and skillset – whilst mastering radiochemistry, radiobiology and in vivo imaging.
Potential Research Placements
- Jonathan Fisher, University College London
- Samantha Terry, King’s College London
- John Anderson , University College London
References
- Fowler et al. Payload-delivering engineered γδ T cells display enhanced cytotoxicity, persistence, and efficacy in preclinical models of osteosarcoma. Sci. Transl. Med. 16, eadg9814 (2024). https://doi.org/10.1126/scitranslmed.adg9814
- Fisher et al. Effective combination treatment of GD2-expressing neuroblastoma and Ewing’s sarcoma using anti-GD2 ch14.18/CHO antibody with Vγ9Vδ2+ γδT cells. OncoImmunology, 5(1). (2016). https://doi.org/10.1080/2162402X.2015.1025194
- Sodji et al. Low Dose Radiation by Radiopharmaceutical Therapy Enhances GD2 TRAC-CAR T Cells Efficacy in Localized Neuroblastoma. bioRxiv 2024.11.02.621668. (2024). https://doi.org/10.1101/2024.11.02.621668
