Harnessing the potential of cancer-on-chip platforms to characterise the impact of radiotherapy on tumour microenvironment
Primary supervisor: Emad Moeendarbary, University College London
Secondary supervisor: Stefaan Verbruggen, Queen Mary University of London
Project
Colorectal cancer (CRC) and glioblastoma (GBM) are aggressive cancers with poor patient outcomes, partly due to radiotherapy resistance driven by tumour microenvironment (TME), which includes factors such as hypoxia, abnormal vasculature, and interactions with stromal cells like cancer-associated fibroblasts (CAFs). Current in-vitro models (2D & 3D) fail to replicate the complexity of the human TME [1], while complex animal models are not suitable to pinpoint the role of specific factors in TME. Animal models differ from human physiology and are expensive, time-consuming, and face ethical concerns, highlighting the need for better, more human-relevant models [2].
This project aims to address these limitations by developing 3D biomimetic-on-chip platforms consisting of microvasculature-integrated with CRC and GBM organoids. These advanced platform will enable to study the effect of radiotherapy on TME in a more realistic TME , providing insights into tumour biology and also mechanisms of radiotherapy resistance.
Aim 1: Establish an advanced vascularized CRC/GBM-on-a-chip model that mimics the tumour microenvironment.
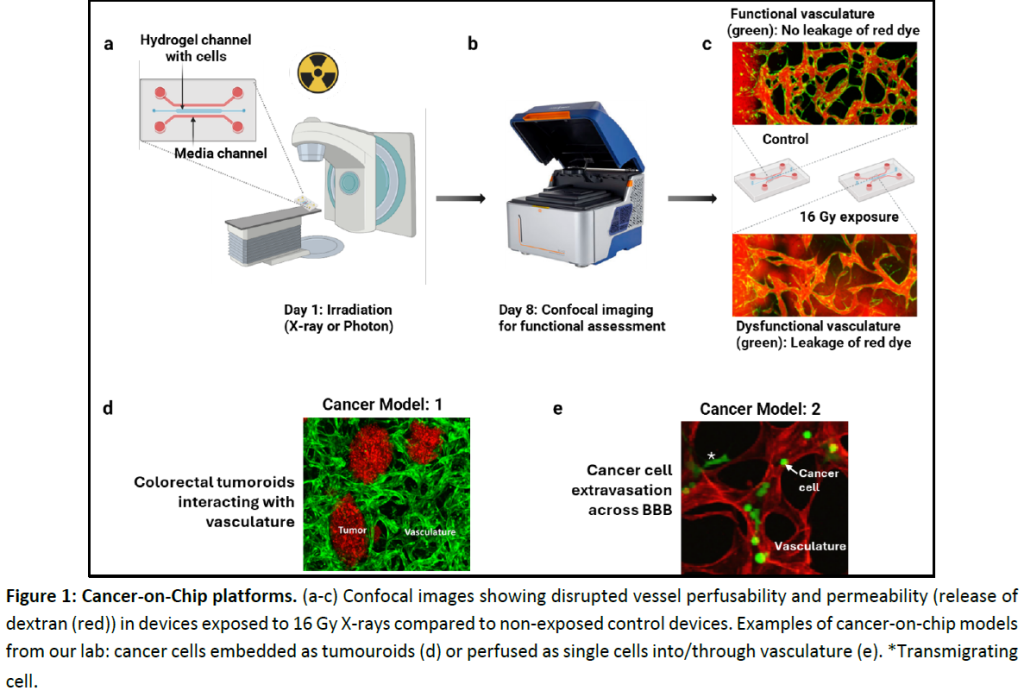
Engineer a microfluidic platform that integrates vascular networks with CRC and GBM organoids to recapitulate the TME to incorporate key features such as perfusable blood vessels, tumour-stroma interactions, and hypoxic gradients. Readout of this aim will include vessel network complexity, perfusability, and permeability (quantified using confocal imaging and machine learning-based image analysis).
Aim 2: Investigate the radiation response and damage in the vascularised CRC/GBM-on-a-chip model.
Using the developed model, we will study the effects of radiotherapy (X-ray and proton therapy) on tumour and vascular components at different doses (0, 2, 8, 16 Gy) and time points (6h, 24h & 7D).
Key experiments before and after radiotherapy to asses changes in:
- Cellular properties (proliferation, migration, and/or invasion, senescence) and DNA double-strand breaks.
- Functional properties (vasculature complexity, perfusability, and permeability) surrounding TME.
- Gene and protein expression profiles using RT-qPCR and western blotting.
- Whole transcriptome by performing RNA sequencing after FACS sorting.
- Vascular stiffness using atomic force microscopy (AFM)
Aim 3: Incorporate CAFs into the vascularized CRC/GBM-on-a-chip model to study their role in radiotherapy resistance.
CAFs are known to play a critical role in modulating the TME and promoting therapy resistance. This aim will involve co-culturing CAFs with CRC or GBM organoids in the vascularised chip model to investigate their influence on radiotherapy response.
Key experiments:
- Determine the impact of CAFs on tumour survival and vascular remodelling post-radiotherapy.
- Introduce T-cells to study its infiltration and its interaction with CAFs and tumour cells.
- Validation of key gene targets using CRISPR/cas9 based activation and inhibition
Candidate Background
A background in molecular and cellular biology, bioengineering, or cancer biology is preferred, not necessary. Experience in 3D-cell culture, microfluidics, or imaging techniques (confocal microscopy) would be advantageous, however, full training will be provided. Candidates with an interest in TME, radiotherapy, or in-vitro model development are particularly encouraged to apply.
Potential Research Placements
- Adrian Biddle, Queen Mary University of London
- Umber Cheema, University College London
- Paul Mulholland, University College London
References
- Rodrigues J, et al. 3D In Vitro Model (R)evolution: Unveiling Tumor–Stroma Interactions. Trends Cancer. 2021; 249-264.
- McAndrews KM, et al. Mouse Models to Evaluate the Functional Role of the Tumor Microenvironment in Cancer Progression and Therapy Responses. Cold Spring Harb Perspect Med. 2024; 14:a041411.
- Micalet A, Cheema U, Moeendarbary E. The bio-mechanical signatures of cancer invasion using biomimetic 3D in vitro models. Tissue Eng Part A. 2023; 29(9-10).
- Whisler J, et al. Emergent mechanical control of vascular morphogenesis. Sci Adv. 2023; 9:eadg9781.
- Javanmardi Y, Agrawal A, Malandrino A, Lasli S, Chen M, Shahreza S, et al. Endothelium and subendothelial matrix mechanics modulate cancer cell transendothelial migration. Adv Sci. 2023; 10:2206554.
