Radiation induced modulation of the extracellular matrix in paediatric solid tumours: implications for CAR T-cell therapy
Primary supervisor: Karin Straathof, University College London
Secondary supervisor: Oliver Pearce, Queen Mary university of London
Project
Unmet need for new treatment approaches for childhood solid tumours. Neuroblastoma and rhabdomyosarcoma are the most common non-CNS tumours in children. Despite multimodal treatment with chemotherapy, surgery and radiotherapy disease relapse occurs in significant proportion of patients. Treatment regimens have been maximally intensified and therefore different treatment approaches are needed to improve outcome for these patients. Immunotherapy with T-cells engineered with a synthetic receptor (chimeric antigen receptor (CAR)) to acquire tumour specificity provides a radically different approach to cancer treatment.
Early promise and barriers to overcome with CAR T-cell therapy for childhood solid tumours. While at an earlier stage of development as compared to haematological malignancies, phase I/II clinical trial data have shown efficacy of CAR T-cells in patients with childhood solid tumours including neuroblastoma [1,2]. However, responses are limited to a subgroup of patients and in many patients relapse still occurs in particular in the context of higher disease burden. Multiple aspects of the tumour and its microenvironment (TME) can impact CAR T-cell efficacy including (i) heterogeneity of target antigen expression, (ii) ability of CAR T-cells to home to and infiltrate into tumour sites [3] and (iii) the presence of cellular, soluble and metabolic factors that can inhibit T-cell function.
Use of radiotherapy to improve access of CAR T-cells to solid tumours. Radiotherapy can alter the TME and may be utilized to render tumours more susceptible to immunotherapies such as CAR T-cells [4]. Case reports of patients with clinical responses when receiving CAR T-cells post radiotherapy support this notion [5]. Ongoing studies in our laboratory show modulation of the TME (increased ratio of M1-like to M2-like macrophages, reduced presence of regulatory T-cells) after a single dose of X-ray irradiation (XRT) in a syngeneic orthotopic model of rhabdomyosarcoma. This translates into enhanced CAR T-cell efficacy after XRT ‘priming’. In addition to modulation of cellular components of the TME, remodelling of the extracellular matrix (ECM) may impact CAR T-cell efficacy. These effects can be direct through cross-linking or fragmentation of ECM components or indirect via stromal cells including fibroblasts and endothelial cells and alteration of their secretion of ECM components and modulating factors. Resulting effects on ECM density and stiffness are expected to impact accessibility of immune cells to the tumour.
This project will focus on the modulatory effects of radiotherapy on the ECM of childhood tumours and will test the following hypothesis: single dose radiotherapy can alter ECM characteristics to achieve improved CAR T-cell infiltration into tumour core resulting in improved efficacy.
Project objectives will include:
• To characterize ECM composition and biomechanical properties in human tumour samples and tumours derived from orthotopic syngeneic models of neuroblastoma/rhabdomyosarcoma (example: Figure 1).
• To study the effects of radiation exposure on these ECM characteristics comparing different dosing schedules and the kinetics of observed ECM alterations using ex vivo and in vivo models.
• To assess the impact of ‘priming’ with selected radiation regimens on CAR T-cell infiltration within the tumour.
• To assess the impact of ‘priming’ with selected radiation regimens on CAR-T cell efficacy.
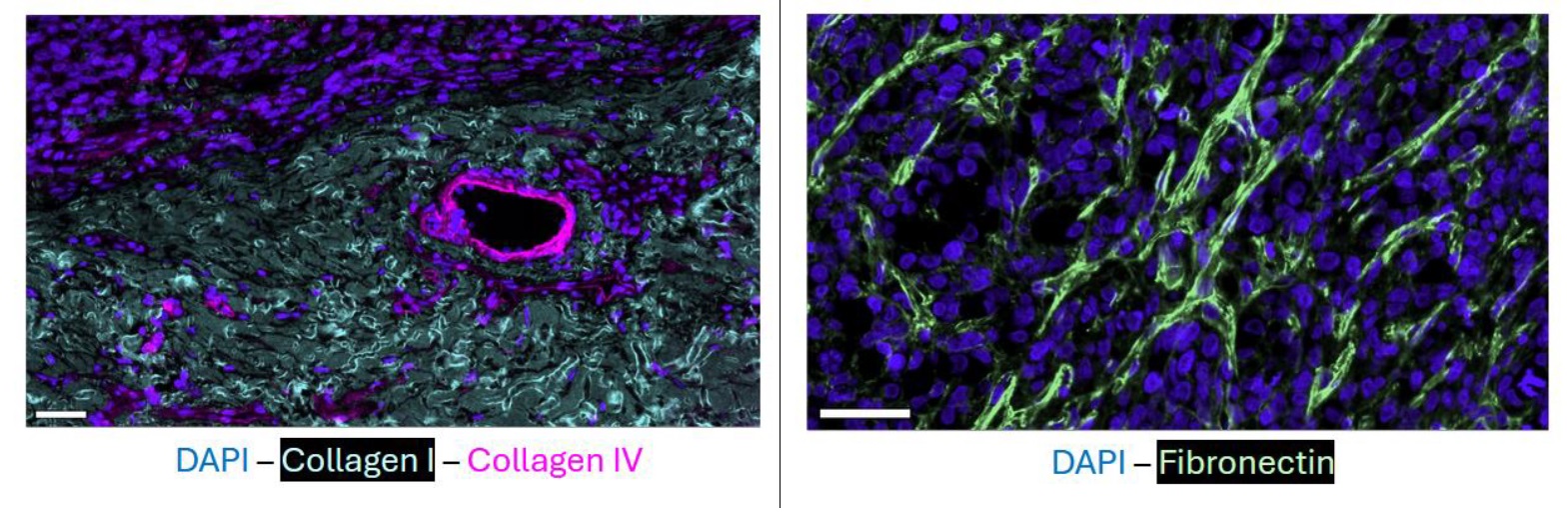
Figure 1. ECM Composition of the rhabdomyosarcoma. Shown are immunofluorescence staining on the MACSima platform for collagen 1, collagen 4 and fibronectin including DAPI reference. Composition as well as biomechanical properties of the ECM of RMS and neuroblastoma will be studied as part of this PhD project.
Candidate background
This project would suit candidates with a background in cancer immunology and tumour biology and an interest in studying the dynamic changes in the tumour immune environment. The candidate will work in laboratory which is integrated in the UCL CAR T-cell programme and is at the cutting edge of CAR T-cell engineering and translation of novel cancer immunotherapies into clinical trials in particular for childhood solid tumours.
Potential Research Placements
- Oliver Pearce, Queen Mary University of London
- Jenny Gains, UCLH
References
- Antitumor activity without on-target off-tumor toxicity of GD2-chimeric antigen receptor T cells in patients with neuroblastoma. Straathof K et al. Sci Transl Med 2020; PMID: 33239386
- GD2-targeting CAR T cells in high-risk neuroblastoma: a phase 1/phase 2 trial. Locatelli F et al. Nat Med. 2025; PMID: 40841488
- Versican Associates with Tumor Immune Phenotype and Limits T-cell Trafficking via Chondroitin Sulfate. Hirani P et al. Cancer Red Commun 2024; PMID: 38517140
- Radiotherapy remodels the tumor microenvironment for enhancing immunotherapeutic sensitivity. Senbo L et al. Cell Death Dis. 2023; PMID: 37833255
- STRIvE-02: A First-in-Human Phase I Study of Systemically Administered B7-H3 Chimeric Antigen Receptor T Cells for Patients With Relapsed/Refractory Solid Tumors. Pinto et al. J Clin Oncol 2024. PMID: 39255444
