Targeting macrophage metabolism to improve radiotherapy efficacy and reduce toxicity in breast cancer
Primary supervisor: Clare Bennett, University College London
Secondary supervisor: Patricia Barral, King’s university of London
Project
Radiotherapy (RT) remains a cornerstone of treatment for breast cancer. Beyond its cytotoxic effects on tumour cells, RT also modulates the tumour microenvironment (TME) and activates immune responses that contribute to anti-tumour immunity. But ionizing radiation induces fibrosis, a common long-term side-effect of radiotherapy limiting quality of life. There is a critical need to develop combined RT with immune-targeted strategies to achieve robust anti-tumour responses whilst minimising toxicities.
Tumour-associated macrophages (TAMs) are the dominant immune cells in solid tumours. Critical regulators of tumour progression, metastasis, and treatment response in breast cancer, their plasticity in the TME makes then attractive therapeutic targets. RT initially activates pro-inflammatory anti-tumour responses from TAMs, but this evolves into a prorepair phenotype which drives fibrosis1. In the skin, we have demonstrated that pro-fibrotic macrophages persist in the post-irradiation leading to long-term “immunological scarring”2.
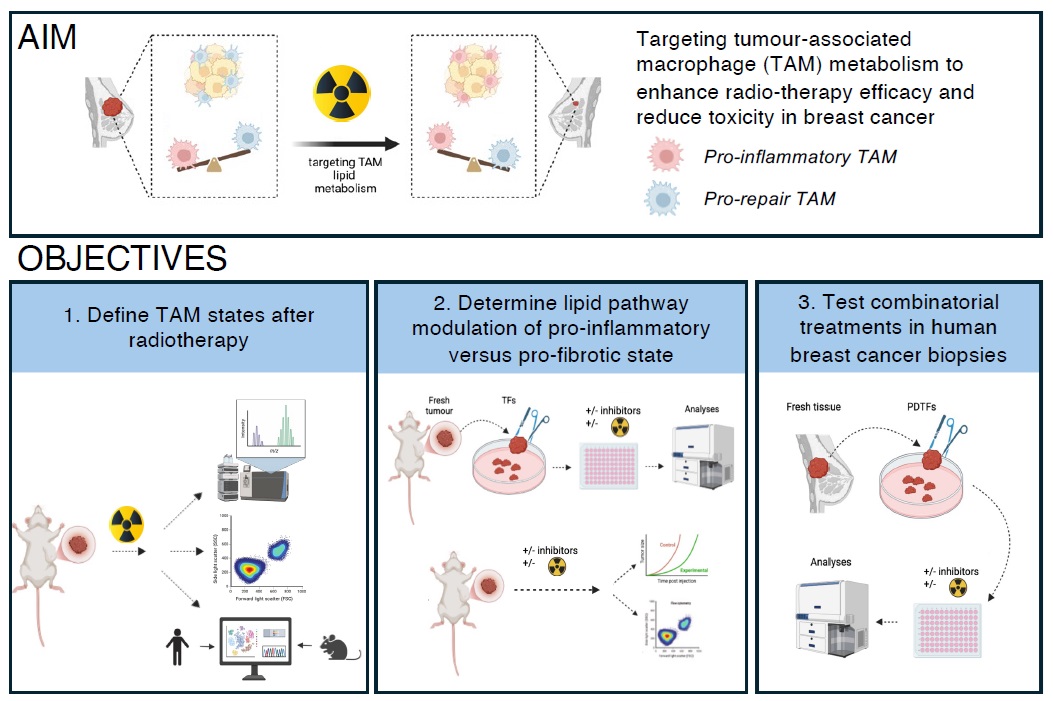
Recent findings from our labs have defined the role of lipid metabolic pathways in determining macrophage state; manipulation of these pathways by blockade of the lipidassociated receptor CD1d switches TAMs in breast tumours to a pro-inflammatory state that is associated with improved patient outcome. Building on these data, this PhD studentship aims to investigate how RT reprograms TAM lipid metabolism and proinflammatory profile in breast cancer. We hypothesise that therapeutic manipulation of lipid metabolism will promote proinflammatory TAM functions and reduce fibrosis, thus enhancing RT efficacy in breast cancer. Our aims are:
Aim 1: To characterize TAM responses to RT we will use murine breast cancer models (EO771, 4T1) established in the Barral lab, and precision X-ray irradiation at the Small Animal Radiation Research Platform (UCL Cancer Institute). We will analyse TAM populations post-RT by flow cytometry, transcriptomics, and metabolomics. Integrated analysis will identify lipid metabolic pathways and species linked to proinflammatory or profibrotic macrophage functions. Comparison with human datasets (e.g. single cell atlas) will be used to determine conserved pathways. Based on these data, availability, and approval
for human use, we will select inhibitors of lipid pathways which will be prioritised for validation in vivo and in human tissues.
Aim 2: To identify lipid pathways connecting metabolism and immunity we will use organotypic ex vivo tumour fragments (TFs) derived from murine models to preserve the native TME and facilitate short-term culture. TFs will be treated with increasing concentrations of lipid pathway inhibitors, alone or in combination with RT. Immune responses will be assessed through profiling of immune cell populations and their secreted factors. Inhibitors that enhance proinflammatory macrophage phenotypes and/or T cell activation in the context of RT will be advanced to in vivo testing. Tumour-bearing mice will be treated with RT +/- inhibitors to evaluate effects on tumour growth, TAM phenotype, lymphocyte infiltration, tissue fibrosis and overall RT efficacy.
Aim 3: To translate findings to human breast cancer we will test the relevance of identified lipid pathways/inhibitors in patient-derived tumour fragments (PDTFs), in collaboration with the CoL Explant and Patient-Derived Xenograft Core (UCL Cancer Institute). Fresh tumour samples will be sourced via the KHP Cancer Biobank, and experiments will be conducted in collaboration with Dr. Sheeba Irshad (consultant medical oncologist, KCL), an expert in immunophenotyping high-risk breast cancers5. Effect of treatment +/- RT on PDTFs will be evaluated by immunophenotyping, metabolic and
transcriptional approaches. These studies will provide proof-of-concept evidence supporting the therapeutic targeting of TAM metabolism in combination with RT to boost anti-tumour immunity in breast cancer.
To know more about the group click here
Candidate background
Motivated candidates with strong backgrounds in cancer/immunology. They will receive comprehensive training in wet-lab techniques (flowcytometry,
metabolic assays) computational metabolomics/transcriptomics, and pre-clinical and clinical tumour models.
Potential Research Placements
- James Macrae, The Francis Crick Institute
- Maria Secrier. University College London
- Sheeba Irshad, King’s College London
References
- Beach et al. The effects of radiation therapy on the macrophage response in cancer (2022)
Frontiers in Oncology https://doi.org/10.3389/fonc.2022.1020606 - West et al. Loss of T cell tolerance in the skin following immunopathology is linked to failed restoration of the dermal niche by recruited macrophages (2022) Cell Reports doi: 10.1016/j.celrep.2022.110819. (Bennett lab)
- Brailey et al. CD1d-dependent rewiring of lipid metabolism in macrophages regulates innate immune responses (2022) Nature Communications doi.org/10.1038/s41467-022-34532-x (Barral lab)
- Bennett and Perona-Wright. Metabolic adaption of mucosal macrophages: Is metabolism a driver of persistence across tissues? (2023) Mucosal Immunology DOI: 10.1016/j.mucimm.2023.06.006
- Gazinska et al. Dynamic Changes in the NK-, Neutrophil-, and B-cell Immunophenotypes Relevant in High Metastatic Risk Post Neoadjuvant Chemotherapy–Resistant Early Breast Cancers. (2022) Clinical Cancer Research doi: 10.1158/1078-0432.CCR-22-0543 (Irshad lab)
