Investigating ²²⁵Ac-DOTATATE as a Novel Combination Therapy for Neuroblastoma Project Description
Primary supervisor: Cinzia Imberti, King’s College London
Secondary supervisor: Jane Sosabowski, Queen Mary University of London
Neuroblastoma is the most common extracranial solid tumour in children. Outcomes for high-risk patients remain poor, with three-year event-free survival just above 50%. Current molecular radiotherapy (MRT) combines the beta-emitter [¹³¹I]MIBG with chemotherapy, but responses are inconsistent, and relapse common. More recently, [¹³¹I]MIBG has been investigated in combination with GD2 immunotherapy, highlighting the potential of MRT combinations.1
MRT with radiolabelled derivatives of the somatostatin analogue DOTATATE represents a promising alternative, as neuroblastomas frequently express somatostatin receptor 2 (SSTR2).2 While beta-emitting [¹⁷⁷Lu]Lu-DOTATATE is under clinical investigation in neuroblastoma3, beta-particle therapy is unlikely to have significant impact on resistant disease. In contrast, alpha particles deliver localised, high-linear energy transfer radiation, with reduced susceptibility to resistance mechanisms.4,5 MRTs based on the alpha-emitter ²²⁵Ac have shown remarkable potential in prostate and neuroendocrine cancers, supporting the rationale to investigate ²²⁵Ac-DOTATATE in neuroblastoma as a novel combination therapy with chemotherapy and immunotherapy.
Project description:
This project will evaluate the therapeutic potential of ²²⁵Ac-DOTATATE in neuroblastoma, building on our expertise in radiotherapy and imaging, including ongoing collaborative research in neuroblastoma models (Figure 1), and drawing on our collaborators’ background in clinical neuroblastoma research (Gaze), and dosimetry modelling (Dickson).
To achieve this, we will use a combination of in vitro and in vivo studies guided by our interdisciplinary expertise (Figure 2).
In vitro testing: neuroblastoma cell lines will be used to assess binding, internalisation, and cytotoxicity of ²²⁵Ac-DOTATATE, alone and combined with chemotherapy/GD2-targeted immunotherapy to identify potential synergies.
In vivo therapeutic studies: efficacy and survival benefit of ²²⁵Ac-DOTATATE will be investigated in mouse models of neuroblastoma. These studies will also explore combination regimens (based on in vitro results) to determine translational potential.
Biodistribution and dosimetry: off-target toxicity due to radiation dose to non-target organs, is a critical barrier to translation and is complicated by the redistribution of 225Ac radioactive daughters after alpha decay, which makes dose and toxicity predictions difficult.5 We will combine ²²⁵Ac-DOTATATE biodistribution studies with preclinical SPECT imaging to track redistribution of daughters (²²¹Fr and ²¹³Bi) and mass spectrometry imaging (MSI) to map bismuth within tissues at micrometre resolution. Integration of these datasets into dosimetry models will provide a more accurate picture of organ doses and identify dose-limiting toxicities, crucial to enable application of the most promising new targeted alpha therapies in children.
Impact:
By demonstrating the therapeutic potential of ²²⁵Ac-DOTATATE in combination with chemo- and immunotherapy, this project will optimise alpha-particle MRT for resistant neuroblastoma. The insights generated will also be relevant to other cancers where ²²⁵Ac-based therapies are already in clinical use, including prostate cancer and neuroendocrine tumours, paving the way for safer and more effective ²²⁵Ac MRT.
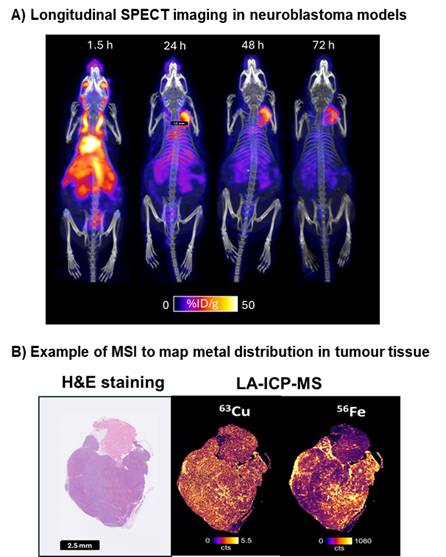
Figure 1. A) SPECT imaging of a mouse bearing a NXS2 neuroblastoma xenograft administered with 111In-labelled anti-GD2 antibody 111In-Dinutuximab Beta. B) Example of MSI to map metal distribution in tumour tissue through laser ablation mass spectrometry (LA-ICP-MS).
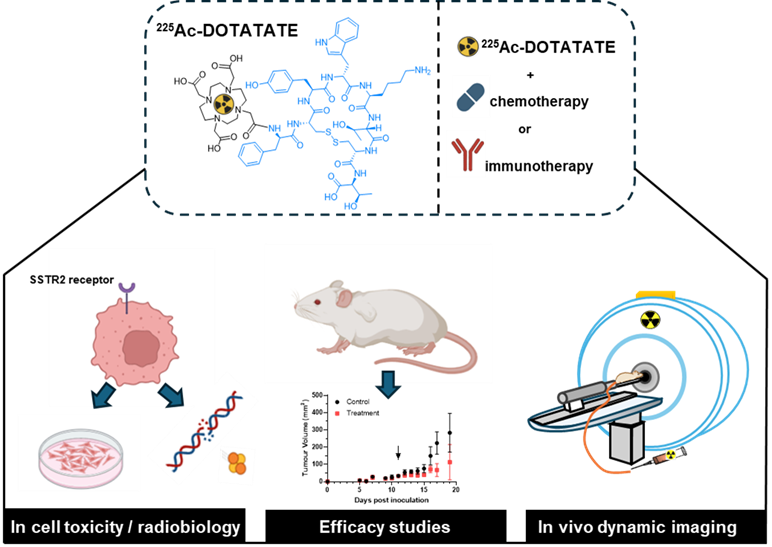
Figure 2. Schematic overview of the project.
Candidate background
Potential Research Placements
- Jane Sosabowski, Queen Mary University of London
- John Dickson, UCLH
- Mark Gaze, University College London
References
- Cash T. et al., “Phase I Study of 131I-Metaiodobenzylguanidine With Dinutuximab ± Vorinostat for Patients With Relapsed or Refractory Neuroblastoma: A New Approaches to Neuroblastoma Therapy Trial”, Journal of Clinical Oncology, 2025, 43, 2490-2501
- Gains. J. E. “Immunohistochemical evaluation of molecular radiotherapy target expression in neuroblastoma tissue“, European Journal of nuclear Medicine and Molecular Imaging, 2018, 45, 403-411.
- Sundquist F. et al., “A Phase II Trial of a Personalized, Dose-Intense Administration Schedule of 177Lutetium-DOTATATE in Children With Primary Refractory or Relapsed High-Risk Neuroblastoma–LuDO-N”, Frontiers in Pediatrics, 2022, 10, 36230
- Rosar F. et al.,” Molecular imaging and biochemical response assessment after a single cycle of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy in mCRPC patients who have progressed on [177Lu]Lu-PSMA-617 monotherapy”, Theranostics, 2021, 11, 4050–4060
- Feuerecker B. et al “Clinical Translation of Targeted a-Therapy: An Evolution or a Revolution?”, Journal of Nuclear Medicine, 2023, 64, 685-692
