Enhancing X-ray and Molecular Radiation Therapy Outcomes by Targeting Senescence
Primary supervisor: Samantha Terry, King’s College London
Secondary supervisor: Sian Henson, Queen Mary University of London
Project
Radiotherapy is a key cancer treatment but often leads to accelerated cellular senescence in irradiated tissues, partly due to mitochondrial damage. Senescent cells remain active and secrete inflammatory factors that promote chronic inflammation, tissue fibrosis, and tumour progression if not cleared. Fibrosis contributes to loss of structural integrity and long-term treatment-related morbidity. Efficient removal of senescent cells is essential for tissue health, but aging-related immune decline impairs this. Thus, radiation-induced senescence, combined with immunosenescence, drives fibrotic tissue remodelling and tumour progression. Enhancing senescent cell clearance and immune function is critical to reduce long-term radiotherapy risks and resistance.
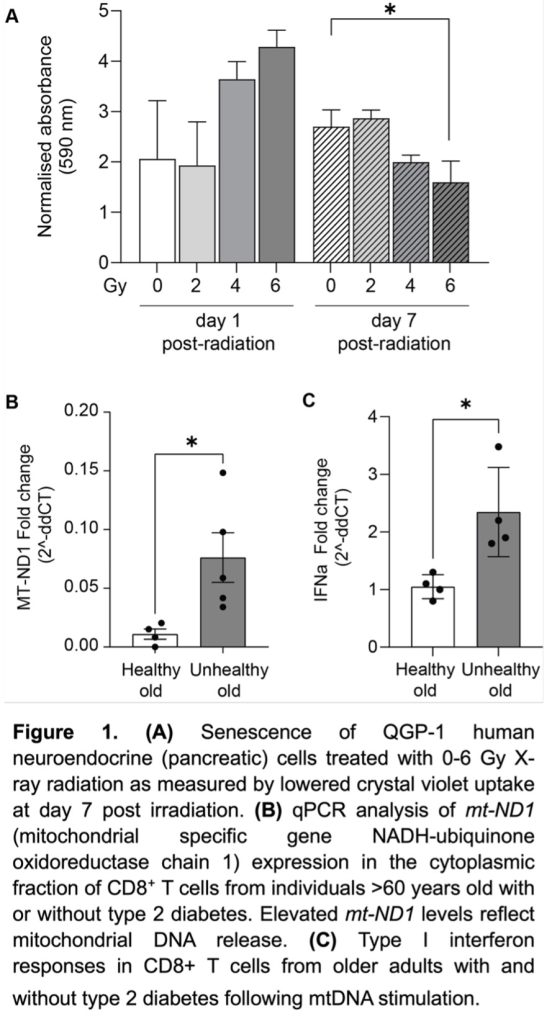
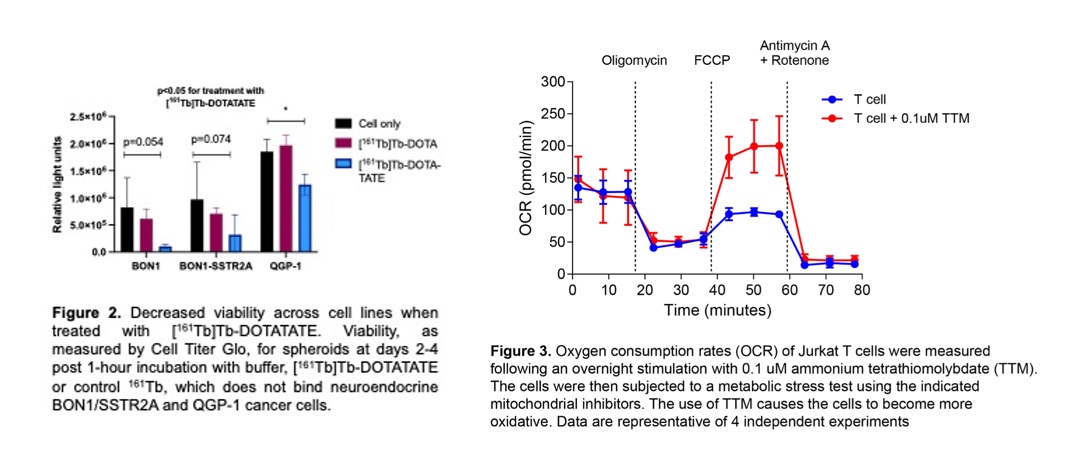
Preliminary data demonstrates that X-ray radiation renders neuroendocrine cancer cells senescent (Figure 1a). We also demonstrate increased mitochondrial dysfunction (DNA release) in the context of unhealthy ageing (Figure 1b) and type I interferon response (Figure 1c); this is important as mitochondrial dysfunction actively contributes to establishment and maintenance of senescence through pro-inflammatory signalling (interferon release). Finally, we can treat neuroendocrine cells with targeted radioactive molecular radiotherapies, e.g. beta particle-emitter [177Lu]Lu-DOTATATE (Reference 1) and beta/Auger electron-emitting [161Tb]Tb-DOTATATE (Figure 2).
Senolytics is a promising therapeutic strategy to clear senescent cells; it also functions as an electron donor enhancing oxidative metabolism and reducing reliance on glycolytic inflammatory pathways (Figure 3). Senolytics directly eliminate senescent cells, while mitochondrial-targeting agent TTM weakens them, making the combination more effective. As such, both approaches combined with radiotherapy could minimise long-term radiation-induced damage or resistance without increasing secondary malignancy risk.
This project utilises X-ray radiation and radionuclides, delivered as established beta particle-emitter [177Lu]Lu-DOTATATE and beta/Auger electron-emitting [161Tb]Tb-DOTATATE. Neuroendocrine cancer cells will be used as a well-established model that is able to be targeted by the DOTATATE vector.
Objective 1: Characterise radiation/radiopharmaceutical-induced senescence and mitochondrial dysfunction in cells exposed to radiation at different absorbed doses. Senescence will be validated using markers such as SA-β-galactosidase activity, p21/p16 expression, and inflammatory mediators (IL-6, IL-18). Mitochondrial dysfunction will be assessed through measurements of mitochondrial mass, membrane potential, ROS production, and mtDNA release. Additionally, metabolic reprogramming will be analysed via Seahorse XF analysis, focusing on glycolysis and oxidative phosphorylation.
Objective 2: Assess therapeutic interventions targeting senescence and mitochondrial dysfunction. Senolytics (e.g., dasatinib, quercetin) and mitochondrial-targeting agents (e.g., TTM) will be tested for their ability to reduce senescent cells and restore metabolic function in cells treated with radiation. Efficacy will be evaluated through cell growth assays, senescence markers, and metabolic assessments.
Objective 3: Studies of radiation-induced senescence, mitochondrial disfunction, and immune profile in tumour-bearing animals determining senescence ex vivo. This work will start with X-ray radiation to determine in vivo dose at which senescence or mitochondrial function occurs. The same radiation dose will then be delivered by radionuclides to determine if the biological effects of radionuclides relating to senescence, mitochondrial disfunction, or immune profiles differ between radiation types of different qualities.
Candidate background
Suitable candidates for this project will have a degree in Biomedical/Biological Sciences. Prior work experiences in a research lab or industry will be considered favourably as will animal work experience.
Potential Research Placements
- Emma Chambers, Queen Mary University of London
- Cleo Bishop, Queen Mary University of London
- Graeme Stasiuk, King’s College London
References
- J Cheng, J Zink, E O’Neill, B Cornelissen, J Nonnekens, L Livieratos, SYA Terry. Enhancing [177Lu]Lu-DOTA-TATE therapeutic efficacy in vitro by combining it with metronomic chemotherapeutics. EJNMMI Res. 2024; 14(1):73.
- KM Wulfmeier, J Pellico, P Machado; AM Carbajal, S Bakker, RTM Rosales, K Sunassee, PJ Blower, V Abbate, SYA Terry. In vitro and in vivo radiotoxicity and biodistribution of thallium-201 delivered to cancer cells by Prussian blue nanoparticles. ACS Appl Mater Interfaces. 2025; 17(9):13577-13591.
- Costa IM, Firth G, Kim J, Banu A, Pham TT, Sunassee K, Langdon S, De Santis V, Vass L, Schettino G, Fruhwirth GO, Terry SYA. In vitro and preclinical systematic dose-effect studies of Auger electron- and beta particle-emitting radionuclides and external beam radiation for cancer treatment. International Journal of Radiation Oncology, Biology, Physics. 2024; 120(4):1124-1134.
- Bystrom J, Da Costa MP, Carrascal-Miniño A, Qureshi A, Keeling GP, Pham TT, Sunassee K, Carroll EC, Garrod-Ketchley C, Schroth J, Tsang VSK, de Rosales RTM, Terry SYA, Henson SM. Impact of age on the homing potential of 89Zr-radiolabelled CD8 + T cells. Sci Rep. 2025 Jul 3;15(1):23801
- Callender LA, Schroth J, Carroll EC, Garrod-Ketchley C, Romano LEL, Hendy E, Kelly A, Lavender P, Akbar AN, Chapple JP, Henson SM. GATA3 induces mitochondrial biogenesis in primary human CD4+ T cells during DNA damage. Nat Commun. 2021 Jun 7;12(1):3379.
